Access to all articles, new health classes, discounts in our store, and more!
Resistance and Susceptibility to Oral Disease: III A Study in Clinical Tooth Mobility and Carbohydrate Metabolism
Presented before the Annual Meeting of the California Dental Association, April 25-28, 1965. Published in The Journal of the California Dental Association, Vol. 41, No. 5, October 1965.
* * *
Introduction
Most clinicians recognize that the same presumably causative factors do not always cause the same degree of disease and, in fact, may yield no pathosis. For example, calculus is regarded by some investigators as a contributor to periodontal afflictions. Yet, the same amount of calculus may be associated with less pathologic response in one person than another; it may be present with no periodontal sequelae in a third person. This clinical constellation is generally explained on the basis of more or less resistance or susceptibility.
Apropos to treatment, many clinicians find that the same or a similar therapeutic technique nets different results in seemingly similar subjects. Thus, scaling may be followed by great reduction in gingivitis in one person, minimal improvement in another and no change in a third patient. These disparate therapeutic responses are often ascribed to differences in host resistance or susceptibility.
All seem to agree that the host state, call it resistance, its antithesis susceptibility, or by some other name (e.g. tissue tolerance), somehow plays a role in the genesis and treatment of disease. What is not clear are the means for measuring host resistance and susceptibility.
This report, a companion to others,1,2 attempts to analyze one method of quantitating resistance and susceptibility through a study of clinical tooth mobility in both a scaled and unscaled environment.
Method and Results
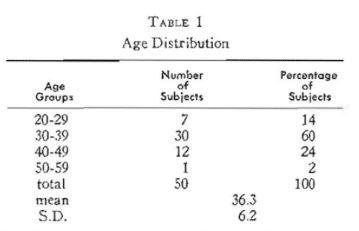
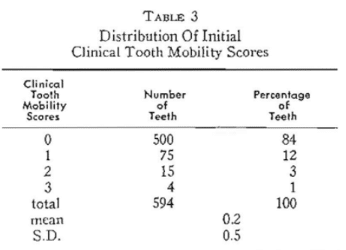
Fifty presumably healthy policemen and firemen (Table 1) participated in this study. At each of two visits (separated by two weeks) clinical tooth mobility was graded (Table 2) for each of the twelve anterior teeth. The theoretical total of 600 measurements (50 subjects x 12 teeth = 600) was reduced to 594 because of six missing teeth. It will be noted (Table 3) that 500 (84 per cent) showed absolutely no clinical tooth mobility. Only one per cent had severe clinical tooth mobility.
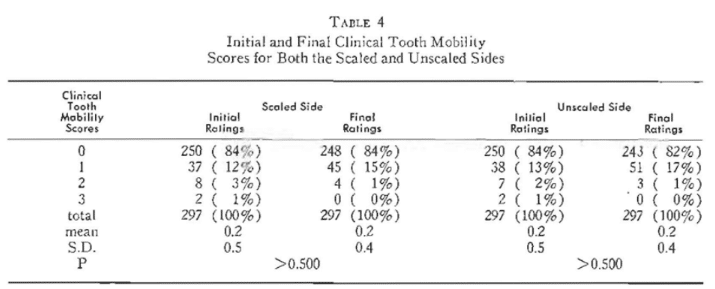
At the initial visit, one-half of the mouth was scaled. The decision to treat the right or left side was done on a random basis. It will be observed (Table 4) that the initial clinical tooth mobility scores for the sides to be scaled versus nonscaled are strikingly similar. For example, 84 per cent of the initial values for both the scaled and unsealed sides are zero. Re-examination two weeks postscaling netted essentially no change as evidenced by the percentage findings and the means and standard deviations which it will be noted are precisely the same. This is underscored by the probability values indicating no statistically significant change.
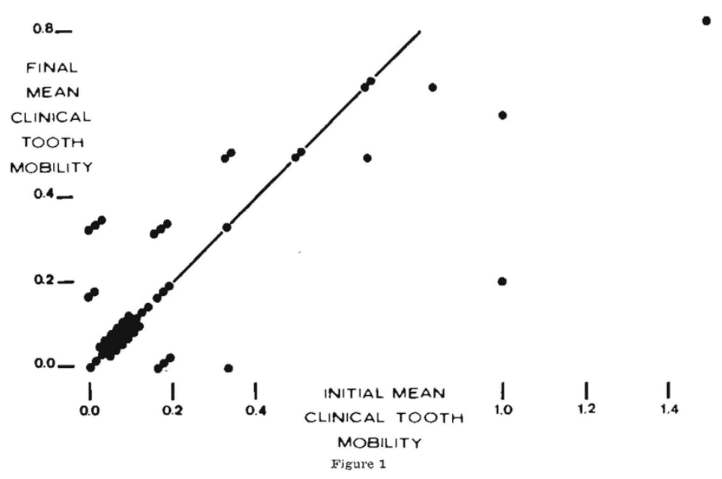
While there are no overall differences in clinical tooth mobility following scaling and no scaling, it is well to view the individual mean clinical tooth mobility scores before and after scaling (Figure 1). Shown on the abscissa are the initial mean clinical tooth mobility scores for the scaled side of the fifty subjects. On the ordinate are the final mean clinical tooth mobility values. It is clear that most scores, specifically 31 of the 50 or 62 per cent, remained unchanged as evidenced by the fact that the values fall precisely on the diagonal line. Ten subjects (20 per cent) demonstrated an increase in clinical tooth mobility by virtue of the fact that these scores fall above the diagonal line. Finally, 9 subjects (18 per cent) appeared below the diagonal line suggesting that the final mean clinical tooth mobility scores were less than observed at the initial visit.
Discussion
It is obvious that different subjects responded differently to the same therapeutic device. Figure 1 shows, for example, four subjects with the same initial mean score of 0.33 who responded differently to scaling (two worsened to 0.50, one remained the same and one improved to 0). The question is why? Certainly, the oral environment was not the same in all of the subjects. Perhaps with other local therapy (e.g., a change in brushing technique or frequency) the clinical response might have been more consistent. Clinical experience says this is likely. But clinical observation also indicates that there may be other, possibly host, factors which are operative.
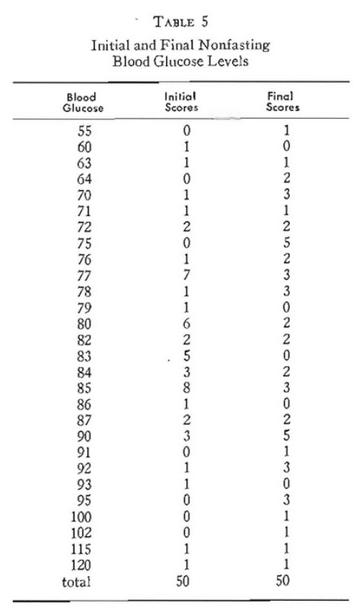
Table 5 lists the initial and final nonfasting (two-hour postprandial) blood glucose levels (Somogyi-Nelson method3-5) for the 50 participants. It is obvious that they range from 55 to 120 mg. per cent. If one grants that 60-100 mg. per cent is the physiologic range (Mosenthal and Barry6), only two are marginally hyperglycemic (115 and 120 mg. per cent) at the initial visit, one is hypoglycemic (55 mg. per cent), and there are hyperglycemic (102, 115, and 120 mg. per cent) at the final visit.
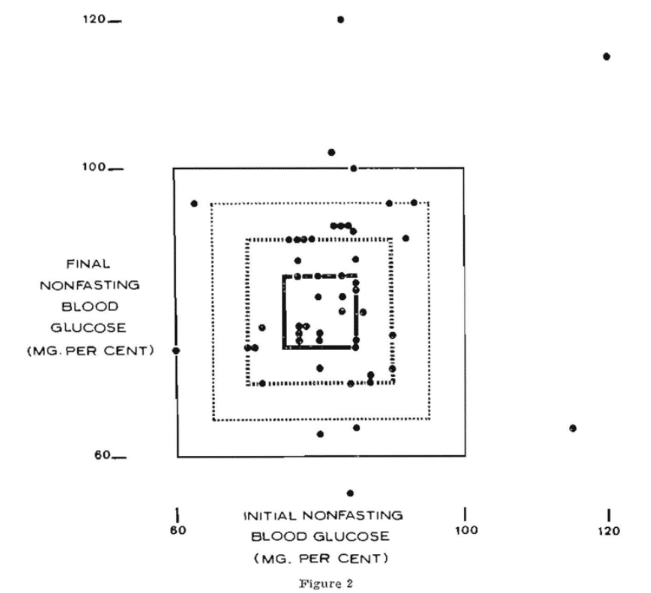
Figure 2 shows the initial blood glucose levels on the abscissa and the final scores on the ordinate. The area within the bold square separates the normal (60=100 mg. per cent) from the pathologic values (outside the square representing below 60 and above 100 mg. per cent). It will be noted that only five of the 50 subjects are plotted outside of the square.
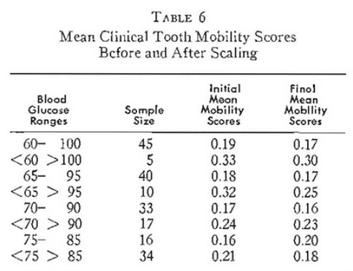
Table 6 shows the means for the two groups before and two weeks after scaling. Three points deserve special mention. Firstly, the mean clinical tooth mobility score for the subjects with the presumably better resistance in terms of carbohydrate metabolism (60-100 mg. per cent) is lower (0.19) than in the group with the poorer host resistance (0.33). Secondly, the so-called better resistance group showed a lower mean clinical tooth mobility score after scaling (0.17) than those with greater susceptibility (0.30). Hence, the evidence suggests that those within the rectangle have less clinical tooth mobility under ordinary circumstances and respond to scaling with a greater reduction in clinical tooth mobility than those outside of the rectangle.
There are a number of investigators7-10 who hold that the present standards for carbohydrate metabolism are too broad. The orthodox approach to establishing physiologic parameters is based on the findings of 95 per cent of a presumably healthy sample. It was thought, therefore, advisable to restudy the observations in the light of other, more restricted, parameters for carbohydrate metabolism.
Figure 2 describes the initial and final blood glucose levels on the x – and y- axes as previously mentioned. However, it will be noted that there is a slightly smaller rectangle representing 65-95 mg. per cent. Utilizing these parameters for physiologic carbohydrate metabolism, it will be noted from Figure 2 and Table 6 that now 40 of the individuals are within the square and 10 outside. Figure 2 also shows progressively smaller boxes representing 70-90 mg. per cent and 75-85 mg. per cent. The clinical tooth mobility findings are summarized in Table 6. It will be noted that, whatever parameters are employed for physiologic carbohydrate metabolism, in all instances the mean clinical tooth mobility score initially for those within the rectangle is less than for those who are outside. This is true utilizing any one of the four rectangles as representative of a delineation between good and poor resistance. Secondly, it will be noted that, as one shrinks the parameter, the initial mean clinical tooth mobility score progressively approaches zero. For example, utilizing the rectangle describing 60 to 100 mg. per cent, the initial mean clinical tooth mobility score is 0.19 and it progressively drops to 0.18, 0.17, and finally 0.16. Thirdly, it is noteworthy that, in all cases but one, the mean clinical tooth mobility score following scaling is lower for the group within the rectangle than outside. This is true with one exception (75-85 mg. per cent rectangle).
On the basis of these findings, it would appear that carbohydrate metabolism may indeed be one reflector of host resistance and susceptibility. Also, these findings suggest that carbohydrate metabolism as a barometer of host state gains importance when the physiologic parameters are restricted. However, it should be pointed out that the relationships here with clinical tooth mobility are nowhere near as sharply defined as previously reported with gingival state1 and periodontometry.2 These observations must not be construed as evidence that a disturbance in carbohydrate metabolism per se causes a lowering in host resistance. It is well known that blood glucose is a function of hypo- and hyperglycemic forces.11-13 If this hypothesis is valid, then changes in the vectors (e.g., diet11-13 and hormones13) should alter blood glucose concentration and such alterations should be paralleled by changes in the clinical state. This, to some extent, is supported by published reports.14-20
Summary
- It is generally recognized in clinical circles that an etiologic factor does not always yield the same, quantitatively or qualitatively, disease.
- It is also generally agreed that the therapeutic technique does not always produce the same end result and, in some instances, yields no beneficial effects.
- In such cases, the discrepancy is frequently ascribed to differences in host resistance and susceptibility.
- An attempt has been made in this report to try to establish one measure of host state through a consideration of the response to oral prophylaxis in patients with different nonfasting blood glucose levels.
- This study confirms earlier reported observations that one can partially explain the response to local therapy through a study of carbohydrate metabolism.
- Obviously, this hypothesis should be checked in terms of other clinical findings analyzed in the light of other biochemical and metabolic measures.
References Cited:
- Cheraskin, E., and Ringsdorf, W. M. Jr. “Resistance and susceptibility to oral disease: I. Studying gingivitis and carbohydrate metabolism.” J. D. Res. (in press).
- Cheraskin, E., Ringsdorf, W. M., Jr., Setyaadmadja, A. T. S. H., and Ginn, D. S. “Resistance and susceptibility to oral disease: II. A study in periodontometry and carbohydrate metabolism.” (submitted for publication).
- Somogyi, M. “A new reagent for the determination of sugars.” J. Biol. Chem. 160: #1, 61-68, September 1945.
- Somogyi. M. “Determination of blood sugar.” J. Biol. Chem. 160: #1, 69-73, September 1945.
- Nelson, N. “A photometric adaptation of the Somogyi method for the determination of blood sugar.” J. BioI. Chem. 153: #2, 375-380, May 1944.
- Mosenthal, H. O., and Barry, E. “Criteria for and interpretation of normal glucose tolerance test.” Ann. Int. Med. 33: #5, 1175-1194, November 1950.
- Cheraskin, E. “Physiologic fasting blood glucose: range or point?” J. Dent. Med. 16, #2, 96-98, April 1961.
- Ringsdorf, W. M., Jr., and Cheraskin, E. “Physiologic cortisone-glucose tolerance test.” J. Med. Assn. St. Ala. 31: #11, 359-362, May 1962.
- Ringsdorf, W. M., Jr., and Cheraskin, E. “Physiologic glucose tolerance test.” Dent. Prog. 2: #4, 281-284, July 1962.
- Ringsdorf, W. M., Jr., and Cheraskin, E. “Biochemical standards of health: Ill. Fasting blood glucose.” New York J. Dent. 33: #5, 185-187, May 1963.
- Brock, J. F. Recent advances in human nutrition. 1961, Boston, Little, Brown and Company.
- Conn, J. W., and Newburgh, L. H. “The glycemic response to isoglucogenic quantities of protein and carbohydrate.” J. Clin. Invest. 15: #6, 665-671, November 1936.
- Soskin, S., and Levine, R. “Carbohydrate metabolism.” 1952, Chicago, University of Chicago Press.
- Ringsdorf, W. M., Jr., and Cheraskin, E. “Periodontal pathosis in man: I. Effect of relatively high-protein low-refined-carbohydrate diet upon sulcus depth.” J. Periodont. 33: #4, 341-343, October 1962.
- Ringsdorf, W. M., Jr., and Cheraskin, E. “Periodontal pathosis in man: II. Effect of relatively high-protein low-refined-carbohydrate diet upon gingivitis.” New York State Dent. J. 28: #6, 244-267, June-July 1962.
- Cheraskin, E., and Ringsdorf, W. M., Jr. “Periodontal pathosis in man: Ill. Effect of relatively high-protein low-refined-carbohydrate diet upon clinical tooth mobility.” Ann. Dent. 22: #1, 13-18, March 1963.
- Cheraskin, E., and Ringsdorf, W. M., Jr. “Stomatology and clinical chemistry.” Ala. Dent. Rev. 9: #2, 7-14, Winter 1961-1962.
- Cheraskin, E., Ringsdorf, W. M., Jr., and Setyaadmadja, A. T. S. H. “Periodontal pathosis in man: XII. Effect of sucrose drinks upon gingival state.” (submitted for publication).
- Cheraskin, E., Ringsdorf, W. M., Jr., and Setyaadmadja, A. T. S. H. “Periodontal pathosis in man: XIII. Effect of sucrose drinks upon sulcus depth.” (submitted for publication)
- Cheraskin, E., Ringsdorf, W. M., Jr., and Setyaadmadja, A. T. S. H. “Periodontal pathosis in man: XIV. Effect of sucrose drinks upon clinical tooth mobility.” J. Dent. Med. 20: #3, 91-96, July 1965.